Crystallography of Biominerals, Presented by Dr. Gabriela Farfan
Synopsis by Andy Thompson, MSDC Secretary
Dr. Gabriela Farfan, the Carolyn Whitney Endowed Curator of Gems and Minerals, described her MSDC Zoom presentation as having two parts: the crystallography of biominerals and recent gem acquisitions at the Smithsonian.
“I will start by giving a little overview of some of my research. And the big question here is: what can crystallography and mineralogy teach us about biominerals? Then, at the end, I am going to give quick highlights of what we have recently brought into the national collection.”
Those two themes reflect her dual role within the Smithsonian’s National Museum of Natural History (NMNH): (1) as a geological researcher who studies the crystal structures and the chemistry of minerals to answer questions in environmental mineralogy, biomineralization and gem science, and (2) as curator of gems and minerals at the Smithsonian Institution, NMNH.
This article focuses on part 1 (biominerals). Part 2 (recent gem acquisitions) is discussed separately in this newsletter.
Readers: The purpose of this article is to highlight some of Dr. Farfan’s key points to encourage you to go to her MSDC YouTube video where you can view her presentation, in its entirety (68 minutes).
Researching the Crystallography and Chemistry of Minerals
Gabriela shared her vision of the role mineralogy plays in today’s world by citing geology professor Dr. Barb Dutrow’s GSA presidential address (2021) in which she said in effect: a lot of people see mineralogy as a science that is no longer relevant, when, in reality, minerals are the basic building blocks that construct our solid earth.
Gabriela affirmed: “Mineralogy is a core science because it’s really at the center of most of these other fields that we think of as rather independent. But, instead, mineralogy is a way that we can translate between seemingly disparate fields,” as illustrated below.
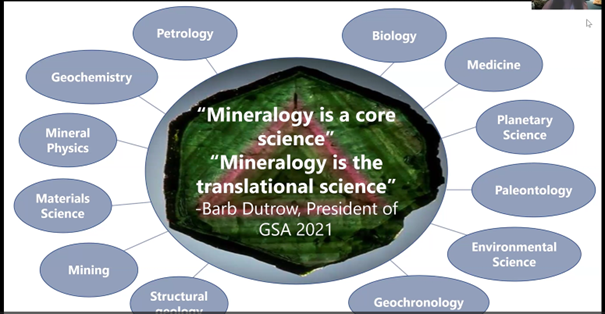
She continued: “Obviously petrologists and geochemists, for example, understand the central role of minerals, but what about the medical sciences, biology, material science, geochronology, environmental science . . . which truly depend on minerals?” Minerals are the subject of their research.
The problem is that too often some scientists study around minerals but do not study minerals as such. “They will often study the biology that surrounds the biomineralogy, for example, but they will not actually take the mineralogical perspective… So it’s really, really important to remind people how important minerals truly are… Minerals really do matter. Mineralogy is a growing and really relevant field.”
Gabriela then reviewed for her MSDC audience some of the basic components of mineralogy.
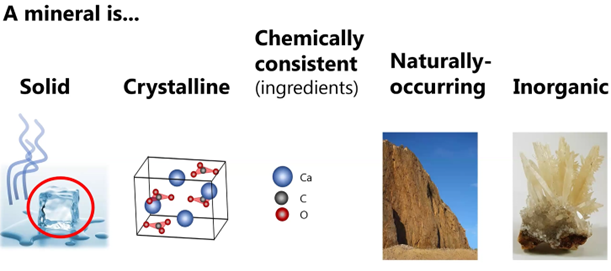
On the last characteristic, “inorganic,” she noted she personally has come full circle and now works as a biomineralogist who studies the crystal structure and chemistry of minerals that are formed under various conditions, including in biological forms.
So, Some Minerals Can be Organic
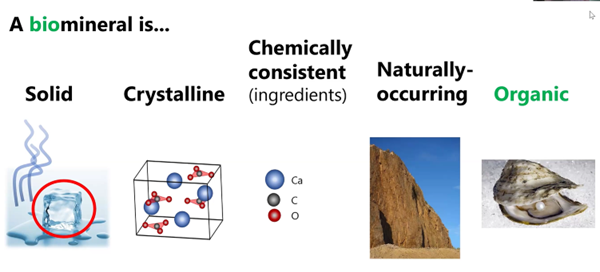
Often times biominerals such as apatite and aragonite shown below in the right-hand column look physically different from their inorganic counterparts in the left column.
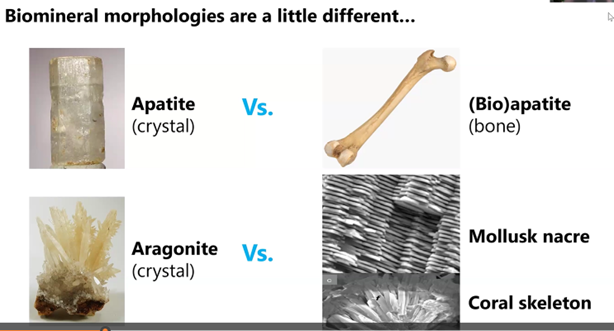
Also, the biominerals can be physically stronger because they incorporate forms of glue. Whereas non-biological apatite crystals, for example, have cleavage planes that can be readily broken by a blow. That is not the case, fortunately for humans, whose bones typically do not readily shatter.
Biominerals are Everywhere
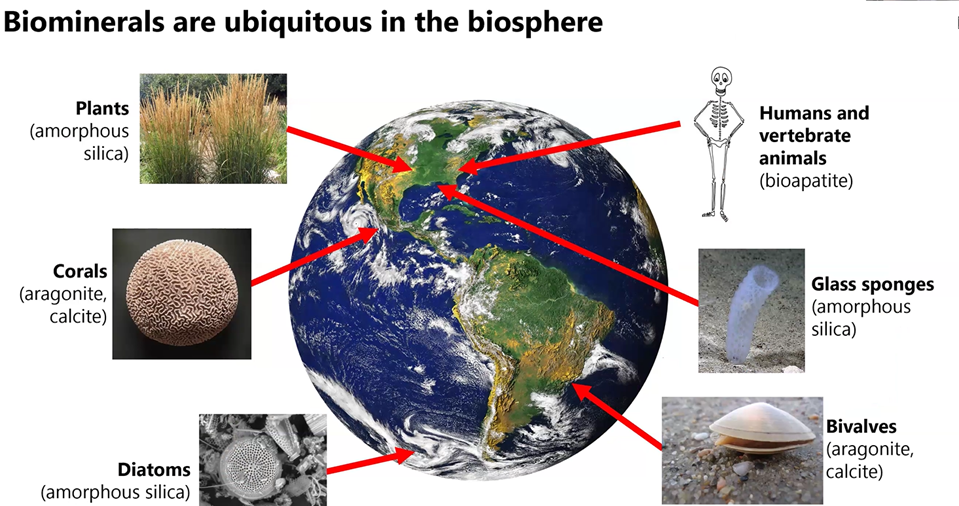
For her presentation to MSDC, Gabriela focused on biocarbonates such as aragonite and calcite that are formed by organisms like coral and bivalves. They are important because they help us understand how carbon gets stored in our world and also because they are so common. Chemically, aragonite and calcite are identical as calcium carbonite (CaC03 ) but they have different crystal structures. Add water or magnesium and you get vaterite or dolomite.
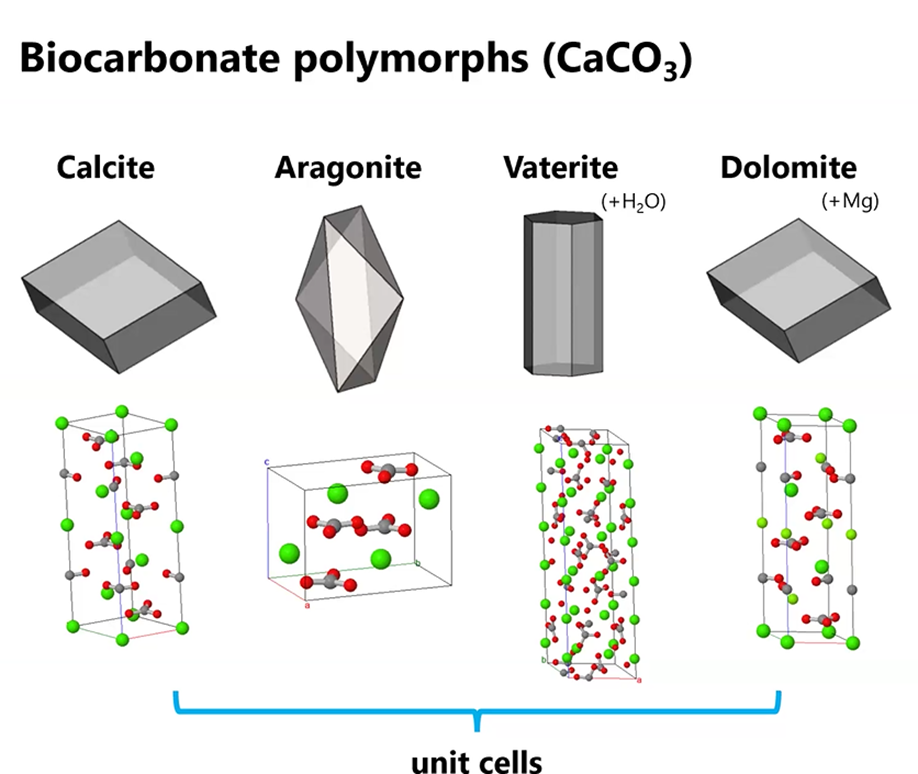
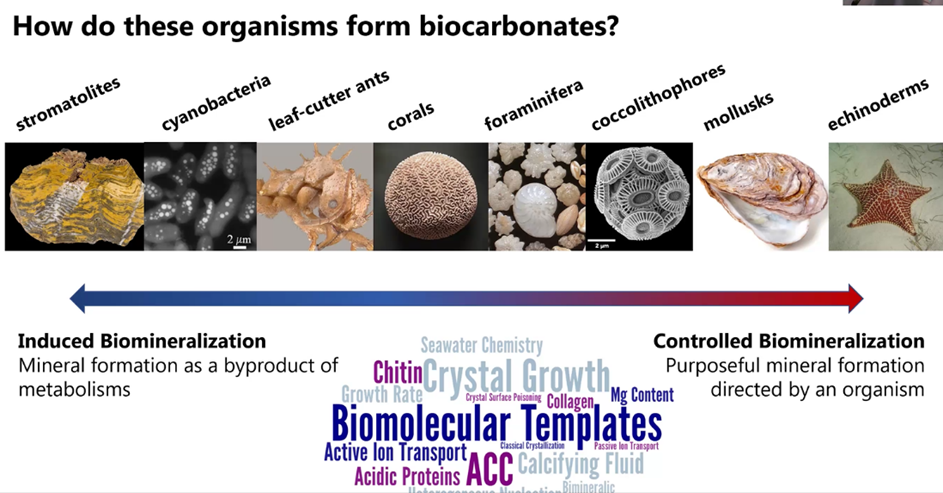
She noted that all the above minerals are commonly found in biocarbonite-forming organisms. But the main focus of her talk is on aragonite.
The organisms to the left of the illustration below form the biocarbonates in a random fashion. But the organisms on the right side, the shell makers, form the crystals in a very structured manner.
All these biocarbonates are important. One example is coral reefs that occupy only 2% of the ocean’s floor space, but support 25% of all marine life. They support the fishing industry, island life, and tourism and provide protection for coastlines.
The coral reefs and other bio-organisms also provide important carbon tracking data and serve as a library for scientists working to understand the past and future of carbon on our planet as shown in the first two circles below.
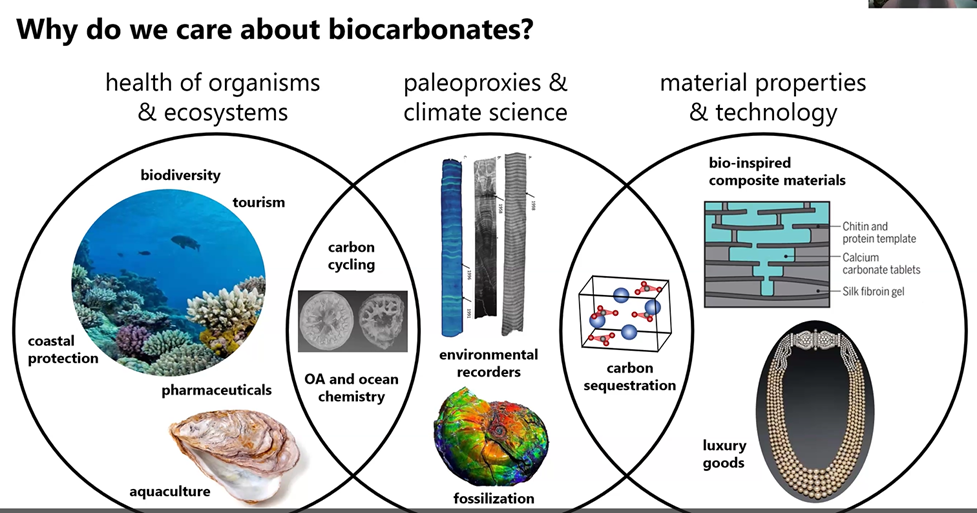
Also, biocarbonates can inspire new forms of technology such as new composite materials (depicted in the circle on the right). “Nacre is incredibly strong and is thousands of times stronger than aragonite on its own and can inspire technology.”
Gabriela described environmental biomineralology as having four dimensions, only two of which have been studied to date. As shown below, “environment” and the “biological processes” have the green check mark indicating they have been studied. But the “interdisciplinary geochemistry” and the “minerals” themselves, on the right-hand side of the illustration, with the red question marks, are frontiers that need to be explored.
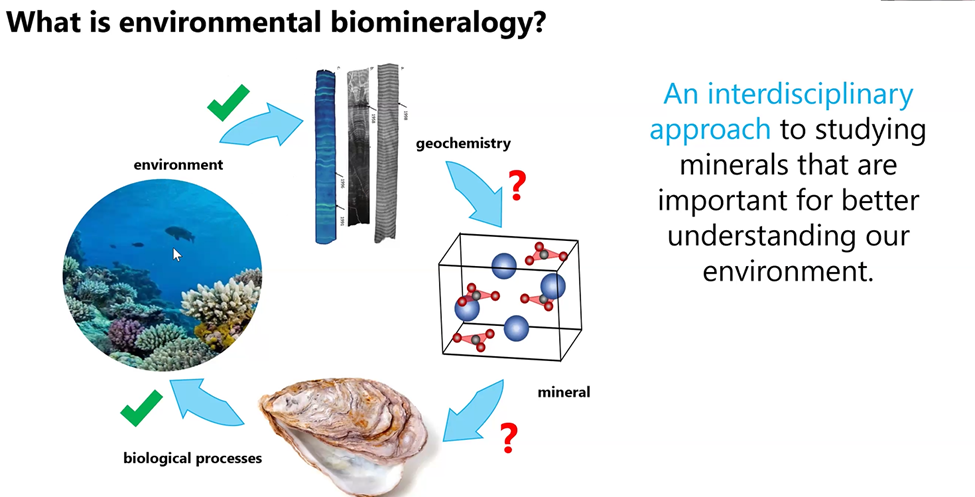

Gabriela then provided several examples of how she has researched environmental biomineralogy in the form of the crystallographic signatures of coral aragonite and the geochemistry and mineralogy of freshwater pearl nacre. Readers can go directly to 34 minutes into Gabriela’s MSDC YouTube presentation to discover her findings to date about the structure of coral and the complex theories of how these “upside down jelly fish” actually grow. She and her coral science colleagues from around the world are working to learn more about the relationship between the very tiny crystal structures and their growth.
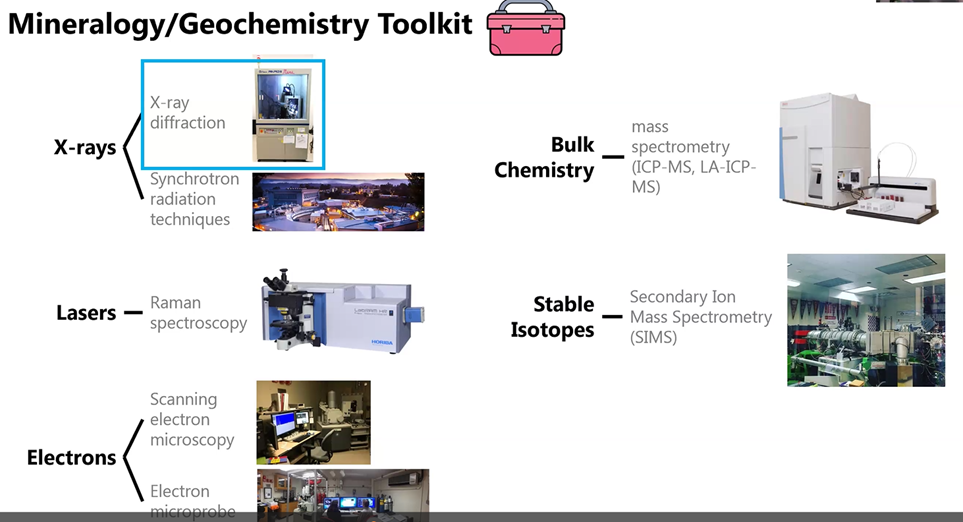
Gabriela also explained the three main tools (X-rays, lasers, and electrons) she and her colleagues use in their research (illustrated below). Watch the video of her presentation to find out which Smithsonian tool is her favorite (hint: What is the Mo Mo?). She said scientists have already identified the fingerprint (X-ray diffraction pattern) for 6,000 of the approximately 9,000 minerals that exist. So this tool provides researchers the mineral identity and a snapshot of the crystal structure that is under investigation.
What the researchers want to know, however, is details about the cell itself, such as its volume. For that, Gabriela explained, they use a second analytic program, the Rietveld refinement analysis which identifies the quantitative description of the organic cell, such as its volume. She shared she is learning that although the general volume of the cells of deep and shallow sea corals are similar, the size of the cell’s A and B axes are significantly different. This suggests to her there could be important differences between how the deep and shallow corals are forming.
What is also fascinating is that the research has positively correlated that the differences in the lengths of the A and B axes of the deep corals compared to the shallow corals depends on the presence of certain trace elements. Readers wanting to focus on these tentative but fascinating findings can learn more by going directly to 43 minutes into the tape.
Gabriela also pointed out several areas needing further research to resolve puzzles. For example, she discovered there were significant differences in the cell sizes of aragonite found in corals versus mollusks, as well as between aragonite formed in beakers filled with sea water vs beakers filled with fresh water. Concluding this review of some of her research, Gabriela said:
“The big take-away is that corals are not as controlled, or they seem to be more on the induced side of the biomineralization spectrum (whereas) mollusks, I would maintain, are still on the controlled side.”
Gabriela concluded this first part of her presentation, coral aragonite crystal structures, with the following summary:
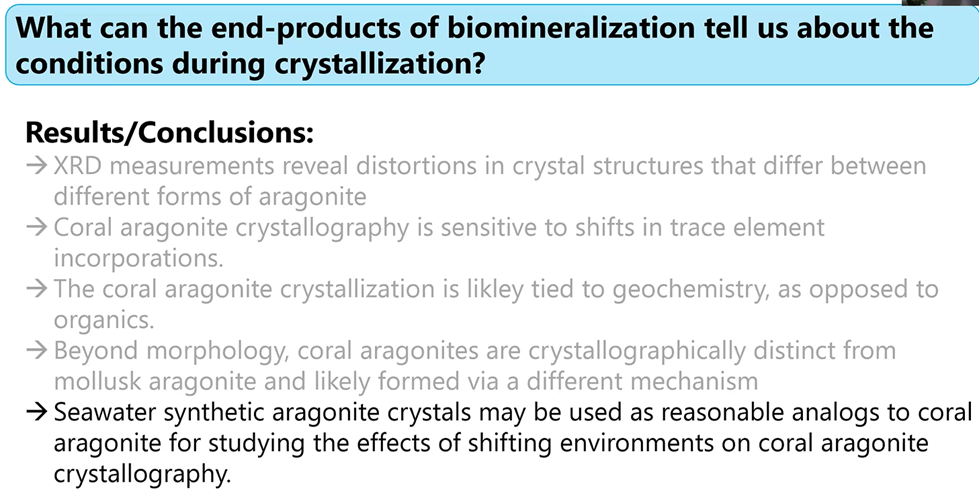
She noted that she and others have been studying the relationship that diverse environmental factors have on the biomineralization processes. Stay tuned because down the road their important findings will be published. She added that she has also been studying pearls which are both a biomineral and a gem.

Readers who want to learn more about Gabriela’s research findings concerning the growth of cultured pearls and their association with diverse variables, such as the water temperature of Kentucky Lake, can go directly to the one-hour mark in her program’s tape: “The pearls are faithfully recording the temperature conditions of the Kentucky Lake.” That finding was not unexpected.
Also, with the help of the Carnegie Foundation, Gabriela was able to explore and find that photoluminescence revealed a trove of new information about the structure of the pearl. She discovered for example that cathodoluminescence highlighted previously unknown traces of elements such as manganese, which appeared as a faint green light in the second column shown below.
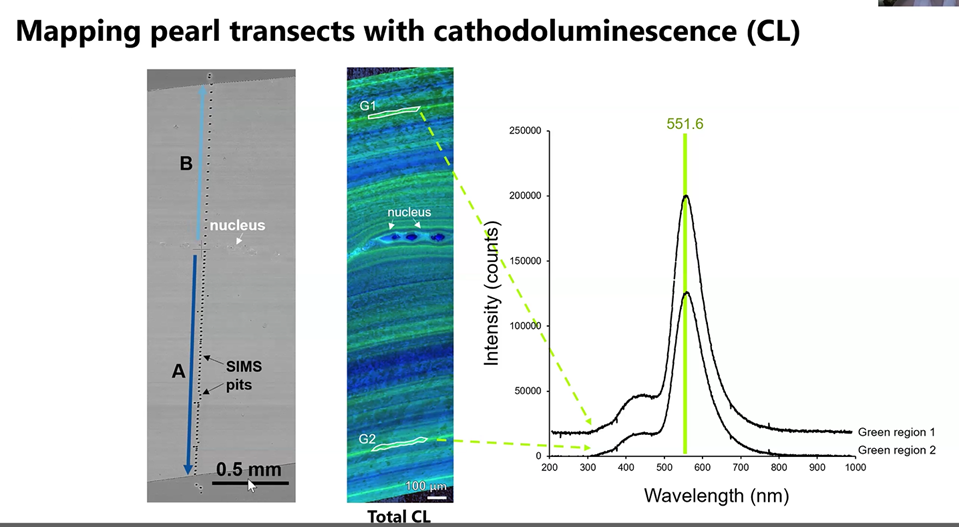
Her research extended further to explore the contrast between the thin green and larger blue aragonite structures. The data obtained from the Raman photon bombardment of the blue crystal (below) suggested to Gabriela there may be important structural differences between the pearl’s green and blue crystals. To discover how the scattering of the light and changing energy levels created a “dance” of the oxygen atoms and how that suggested the manganese was incorporated into the pearl during the Fall season, go directly to the 68-minute mark of her YouTube video.
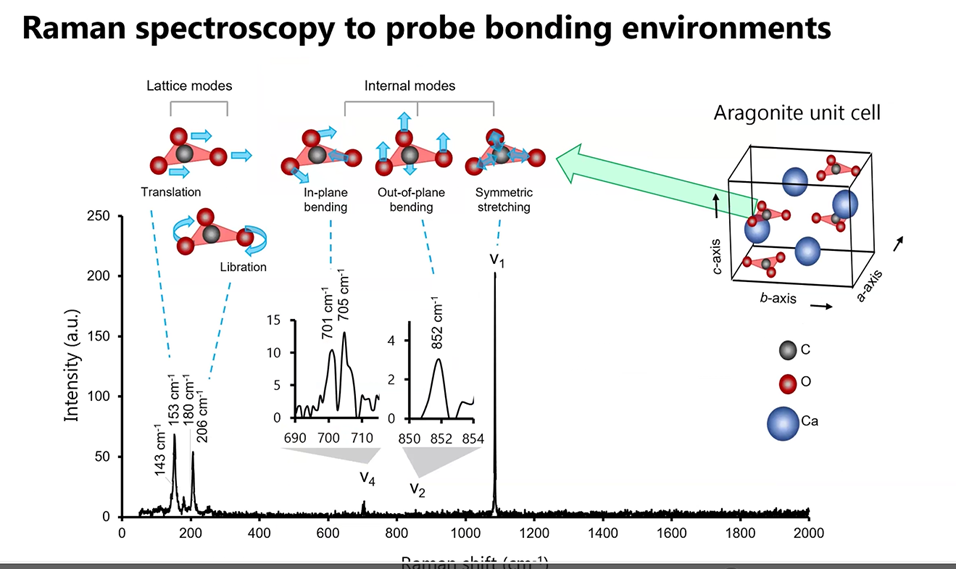
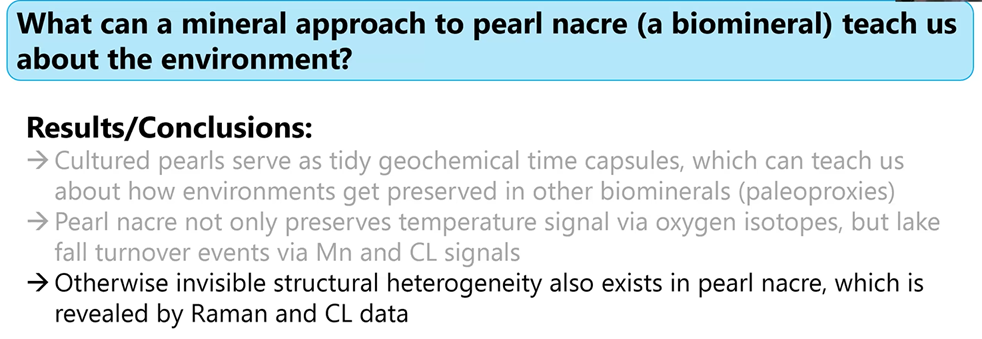
Gabriela noted: Look at how much more we can learn by combining these tools that typically have not been extensively used in the study of mineralogy. She then highlighted some conclusions to her mineralogical research with what pearl nacre can teach us about the environment.
Gabriela expressed her thanks to all her collaborators, many noted below, including institutions and students, who helped make this interdisciplinary approach to mineralogy possible.

