Collecting Fluorescent Minerals from Franklin, NJ by James Van Fleet
Synopsis by Andy Thompson, MSDC Secretary
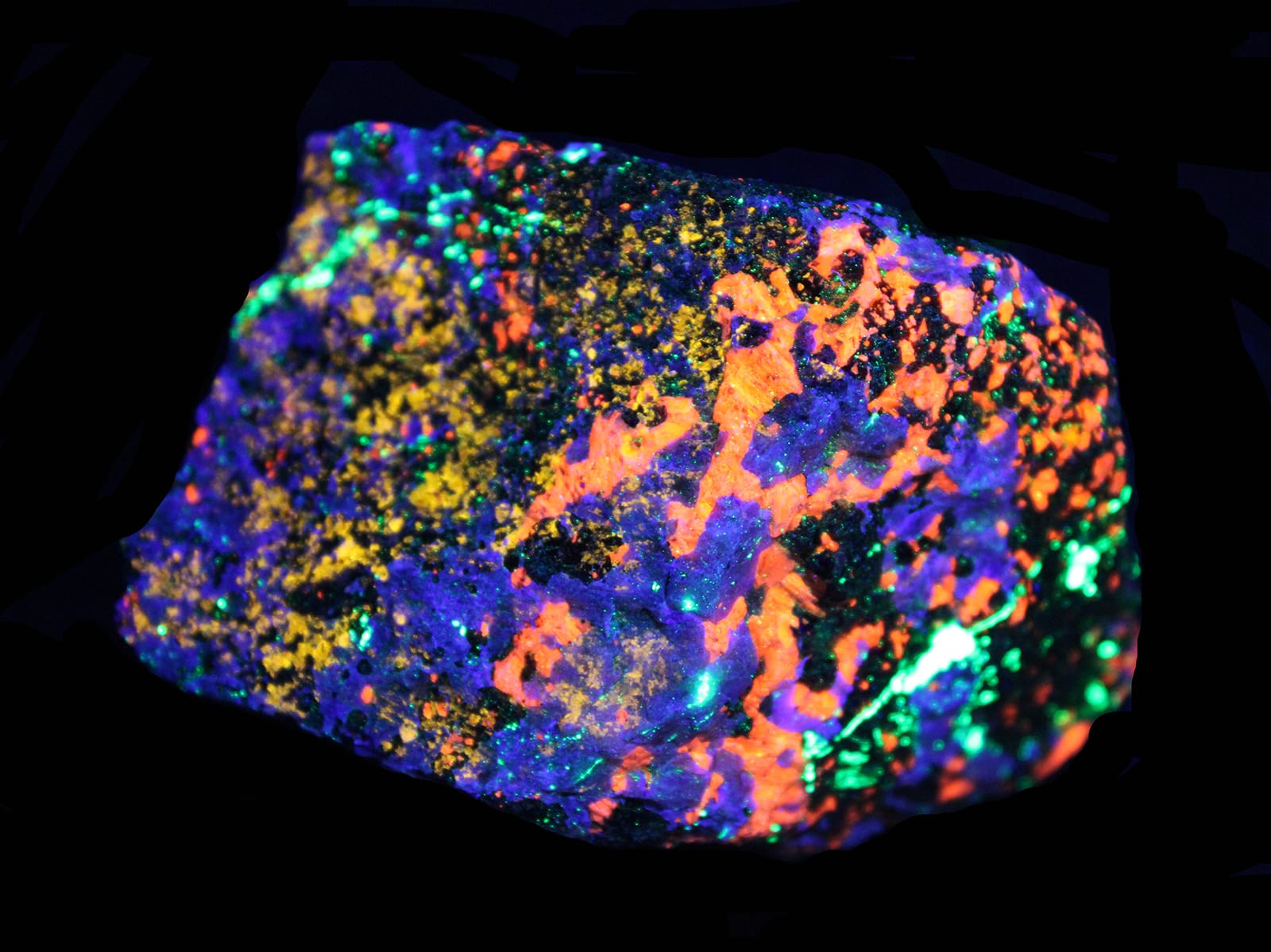
Readers: The purpose of this report is to give those who missed it an overview and to encourage everyone to watch the video of his excellent presentation. Here is the link: Collecting Franklin Fluorescent Minerals – James Van Fleet (52 minutes).
Jim began his presentation to MSDC members by providing a map showing the location of the Franklin and Sterling Hill Mines, whose beautiful and diverse fluorescent minerals were the focus of his talk. These two mines, Jim said, separated from each other by 1.5 miles, together constitute “the fluorescent mineral capital of the world, according to the New Jersey legislature.”
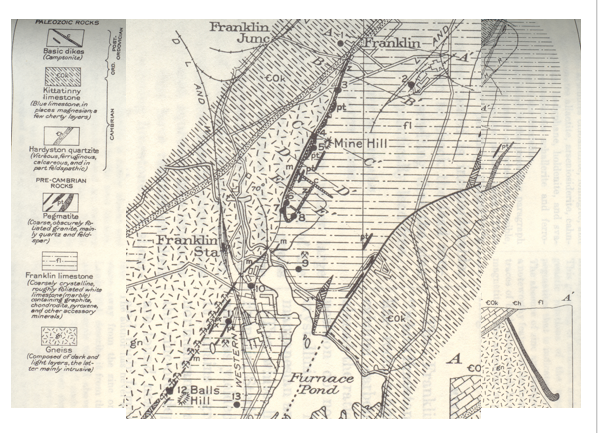
Jim shared the illustration below showing the very diverse geological formations surrounding the mines. It includes Paleozoic dikes of the post-Ordovician era, the Ordovician Kittatinny blue limestone, Cambrian hardyston quartzite, Precambrian pegmatite and Franklin limestone.
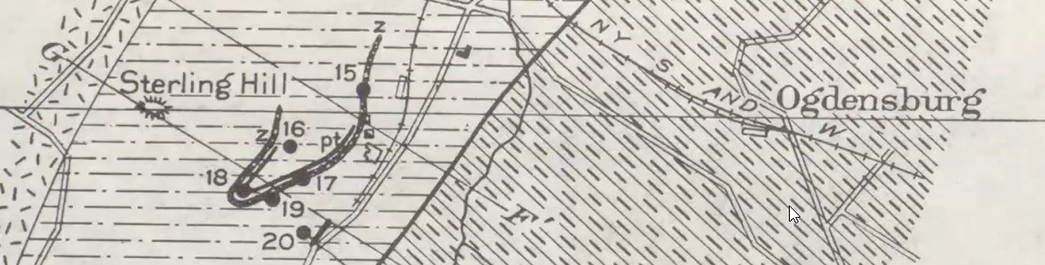
By watching the video and Jim’s cursor, viewers will learn the primary sites where the mining took place, such as where the limestone completely surrounds the ore body, illustrated by the dark line with the hook on the bottom. There, and at the “Mine Hill” and along the row of tiny mine icons (hammer and sledge in the shape of an X), were productive iron mines. All those mines were at contact points between the gneiss and limestone.
Early Smelting Problems
In the early history of the mining, in the 1740s, the miners ran into smelting problems. The first 70 or so years were characterized by extracting iron without the recognition that the ore also contained zinc. As a result, for many decades, the smelting process produced problematic globs of soft mixed metals, known as “salamanders,” which damaged the limestone furnaces. Consultation with chemists in England finally identified that the problem was the miners unknowingly were working not with pure iron, but also with zinc.

Sterling Hill, the second of the two mines famous for producing fluorescent minerals, is located 1.5 miles to the south of the Franklin mine was also is embedded in limestone. Initially, misidentification of minerals was also a problem there.
Folklore reported that the Dutch in the 1640s noticed the red colored ore outcrops which they initially reported as copper. But over time, the mining of iron ore proceeded mostly without problems and continued production for the next three centuries. In 1986, tax problems with the local township required shutting down the Sterling Hill mine.

Jim shared several photos of the interior of the Sterling Hill Mine, as well as the outdoor surviving mine structures pictured above. In the photo on the left, the tall grey structure shows ground level rectangular openings where rail cars came through to be filled with the raw zinc ore from the cylinders above. In the photo on the right, at the base of the rock wall are pale green doors of the adit, the horizontal tunnel leading to the mining operation within the deep rock formation.
Subsequent to the mine’s closing, the miners’ changing room was converted into a museum which, along with the Franklin Mineral Museum received high praise from its visitors, including many MSDC members who visited the sites.

The Fascinating Nature of Fluorescent Minerals
The primary focus on Jim’s presentation, the fascinating nature of the two mines’ fluorescent minerals, he attributed to the uniqueness of their minerals, summarized in the following slide.

These two mines are the only sites in the world where zincite, franklinite, and willemite are the primary ore minerals. That is what draws mineral collectors to these sites to dig for specimens in the tailings.
In the 100-plus photos Jim generously shared of those minerals, his point was crystal clear in that many showed contrasts of how specimens appear in sunlight and then under shortwave, longwave, and some under longwave UV LED light. These mines are unique, Jim said: “Most of the world’s zinc mines are actually sphalerite” (zinc sulfide).
Jim’s show of the mines’ minerals began with “Everybody’s favorite, my favorite, and Kenny’s (Reynolds) favorite is willemite.” Here is willemite as it appears under natural sunlight:

But under shortwave UV light, below, it looks as follows:

Then, illuminated with longwave UV light below, it appears with greater brightness.

Jim noted that willemite is called “gemmy” because it can be cut and facetted into gemstones.

But the cabbed and facetted gems then fluoresce beautifully when hit with UV light.

Jim showed examples of willemite with small inclusions of red zincite and black franklinite, as well as variations of willemite with mineral inclusions of many colors (e.g., light green, mint, purple grape, yellow, brick red, tan, pink, dark brown, gray, and black), all of which fluoresce green. Of all of these, Jim noted, “I think Ken Reynold's favorite is the white willemite with acyclic needle-like radiating willemite crystals that fluoresce brilliantly. And, they also phosphoresce.”

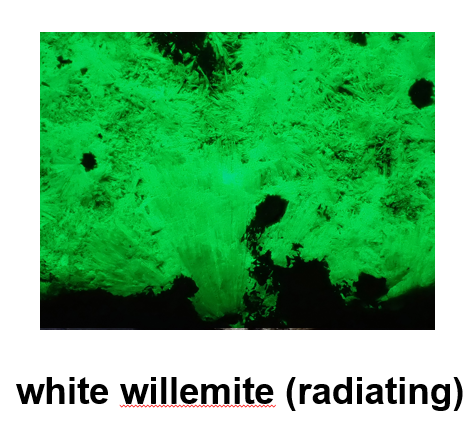
Jim explained that the above specimen and “other willemite specimens may fluoresce, continue to glow for hours, after you take away the UV light.”
Jim then moved on to other minerals found at the two mining areas. Jim noted that “calcite is the most common skarn mineral in Franklin and Sterling Hill sites.” Below, for example, is a salmon calcite as it appears in sunlight and then under UV light.


Below is “Crazy Calcite” from Franklin which is a combination of calcite and dolomite, before and after UV light. The tiny black specks are Franklinite, also known as “pepper.”

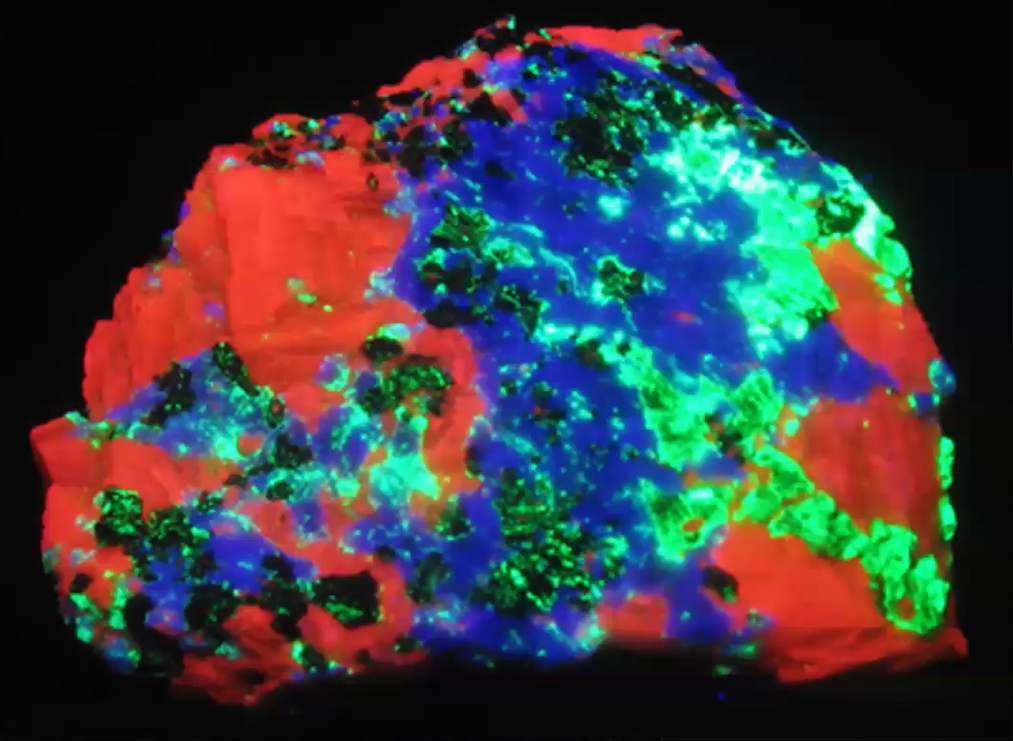
Unusual Color Combinations
Jim shared photos of many additional minerals such as hemimorphite, microcline feldspar and hardystonite (shown below), whose diverse colors beg for an explanation.
Using another hardystonite specimen below, Jim explained how during the process of these rocks’ formation, the tephroite and willemite interaction is such that “the tephroite (appearing to fluoresce green) can’t accept the zinc silicate and so the willemite is literally squeezed out (exsolved) and forms little dots or lines.”
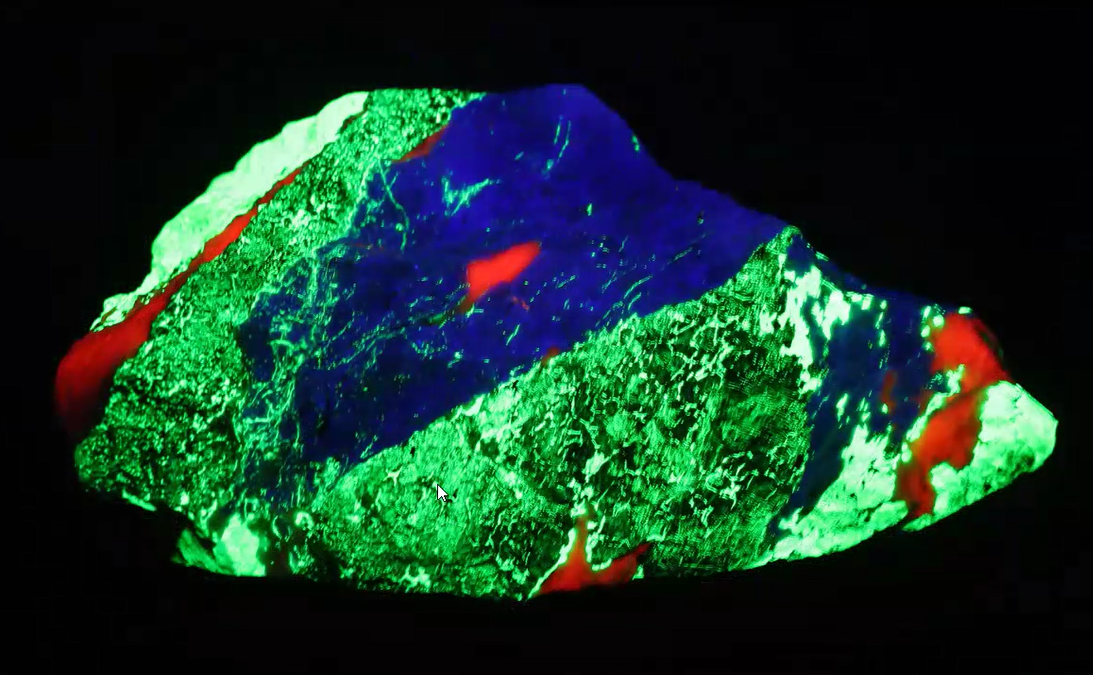
“There’s some interesting geology going on beyond the fluorescence” Jim noted, in reference to a clinohedrite specimen shown below. This is when interested readers will definitely need to view the YouTube video of Jim’s explanation in which his cursor points out the behavior of the diverse minerals and what brought them to be where they came to rest in the specimen.
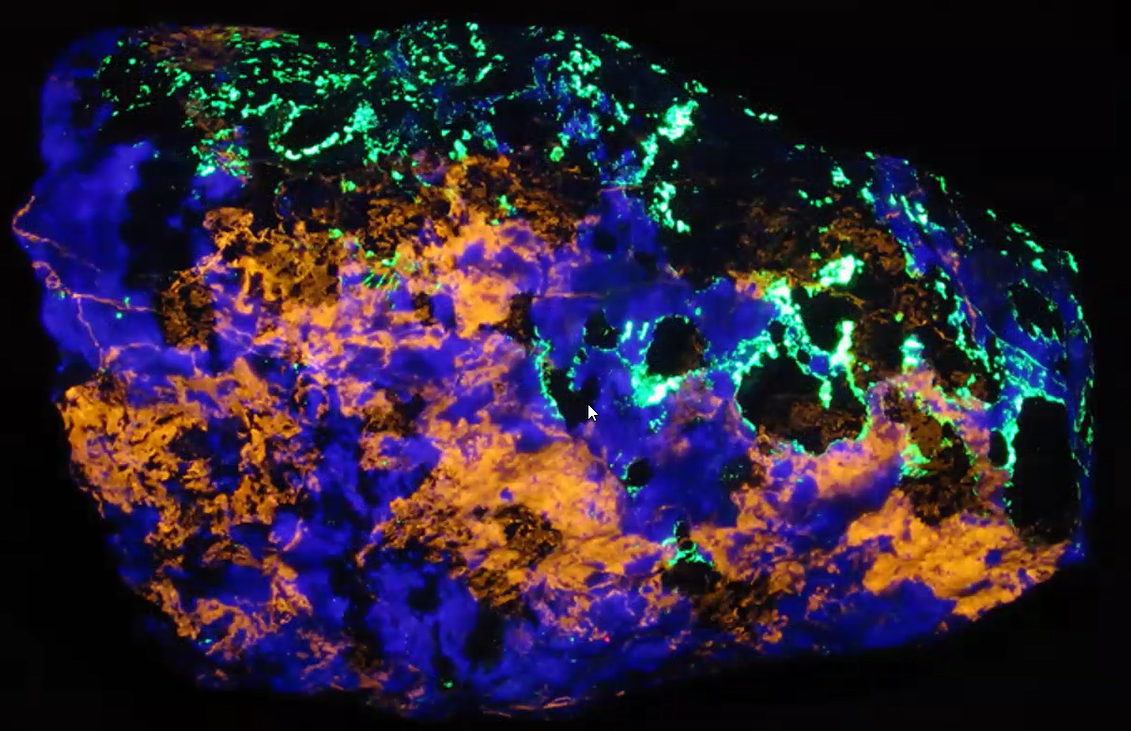
Apatite Minerals
After showing many additional specimens of willemite interacting with other minerals, Jim introduced another type of a fluorescent mineral group, the apatites, which has at least five confirmed species.

Hedyphane
One of the five of this “most complicated” apatite mineral supergroup is hedyphane which is the lead apatite.

Above is hedyphane viewed under normal sunlight. Below, under mid-wave UV light, the lead apatite fluoresces an orangey red.

Turneaureite
The rarest of all the apatites found at Franklin is turneaureite, shown below. “It is usually found in a sand calcite to salmon calcite matrix.”

As seen below, it fluoresces a really bright orange.

One mineral stands out as unique for a peculiar reason. Scheelite, shown below, will always fluoresce under UV light. Unlike some minerals, given its purity, its fluorescence does not depend on any impurities or inclusions. [During WWII, prospecting for scheelite (tungsten ore) to support the war effort created a demand for UV lamps and really boosted the fluorescent mineral hobby.]
Scheelite
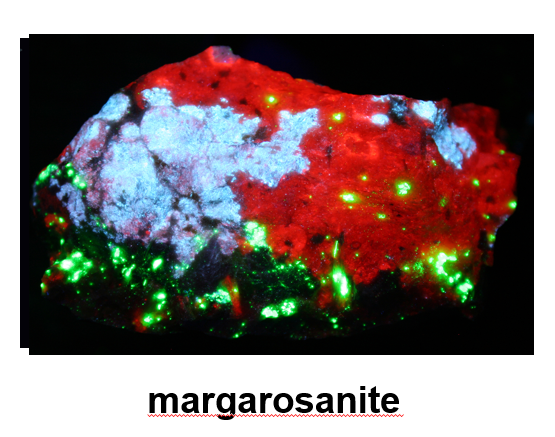
Margarosanite
Pictured below in sunlight, this mineral is “everyone’s favorite’ (and is) hard to come by and pricey these days.”

And below, fluorescing under UV light, “the sky blue is really distinctive.”
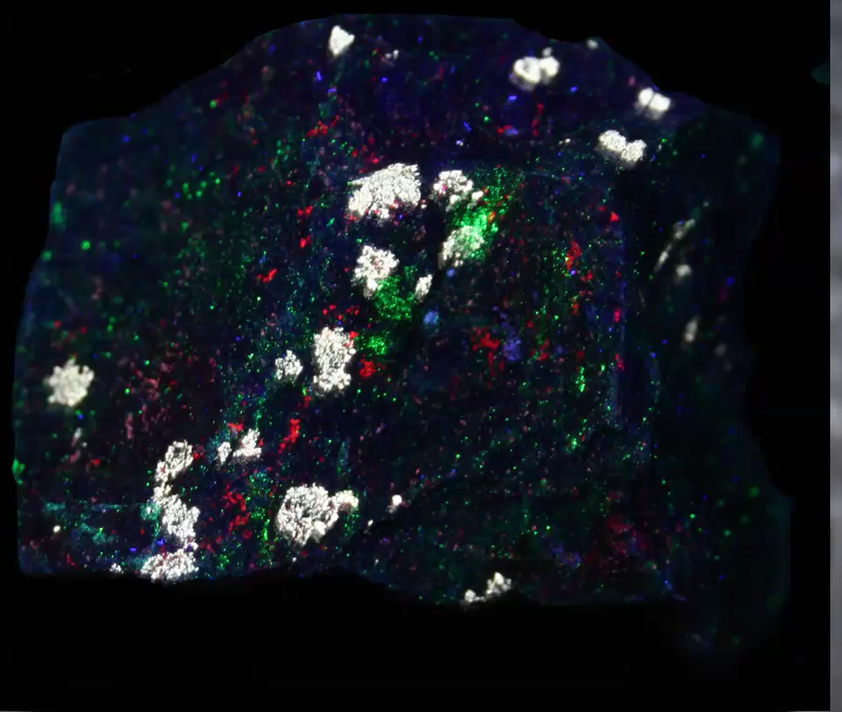
Jim showed another dozen or so minerals and noted that sometimes tiny specks of willemite dust are pervasive. So a specimen can appear to be fluorescing but it is not actually doing so. Below is a case in point. The native copper appears to fluoresce but it is only the willemite dust on the surface that is fluorescing.


Curious Geological Features
Fluorescent minerals sometimes can show evidence of their earlier formation and alterations, and Jim presented the Sterling Hill mylonite specimen below as an example. It shows signs that it had been flattened and stretched by faulting which occurred throughout the wider terrain where it was found.

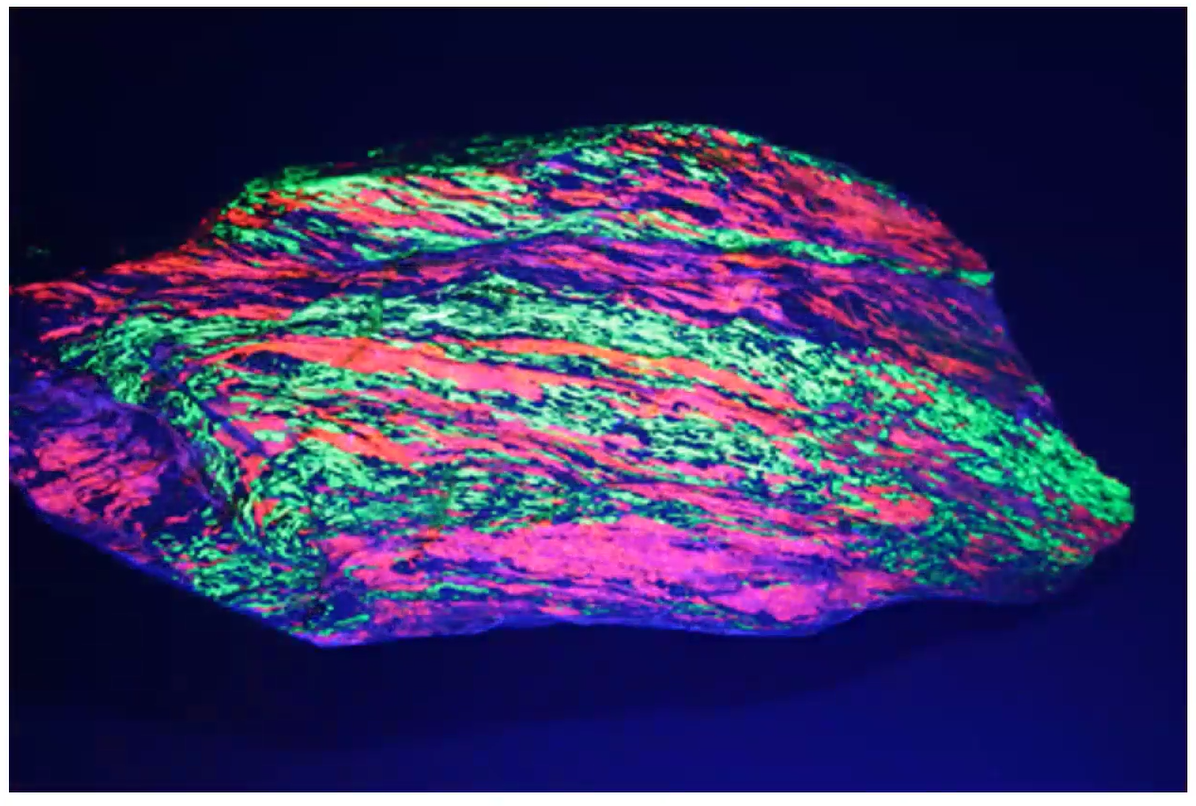
The “circuit board willemite” below, from Sterling Hill, exemplifies another type of alteration identified as a “tephroite exsolution lamellae.” This happens when the plate-like crystals of Willemite have been squeezed out of the tephroite host rock in two phases as the rock cools making thin green parallel lines (lamellae).

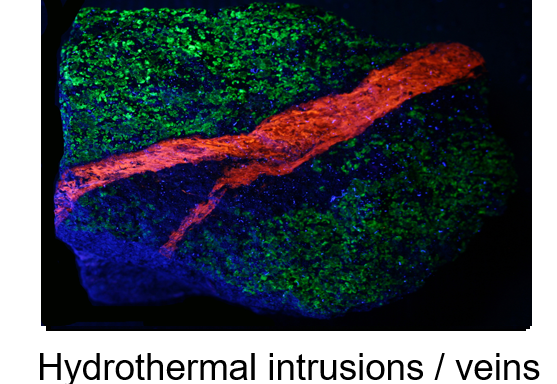
And below is a more common event where the longwave UV fluorescence shows a heated hydrothermal sphalerite fluid (red) had intruded into the host rock (green) and quenched the fluorescence on either side (dark blue) of the intrusion zone.
UV Flashlight Recommendations
Jim then recommended a particular variety of UV flashlight for use in creating fluorescence:

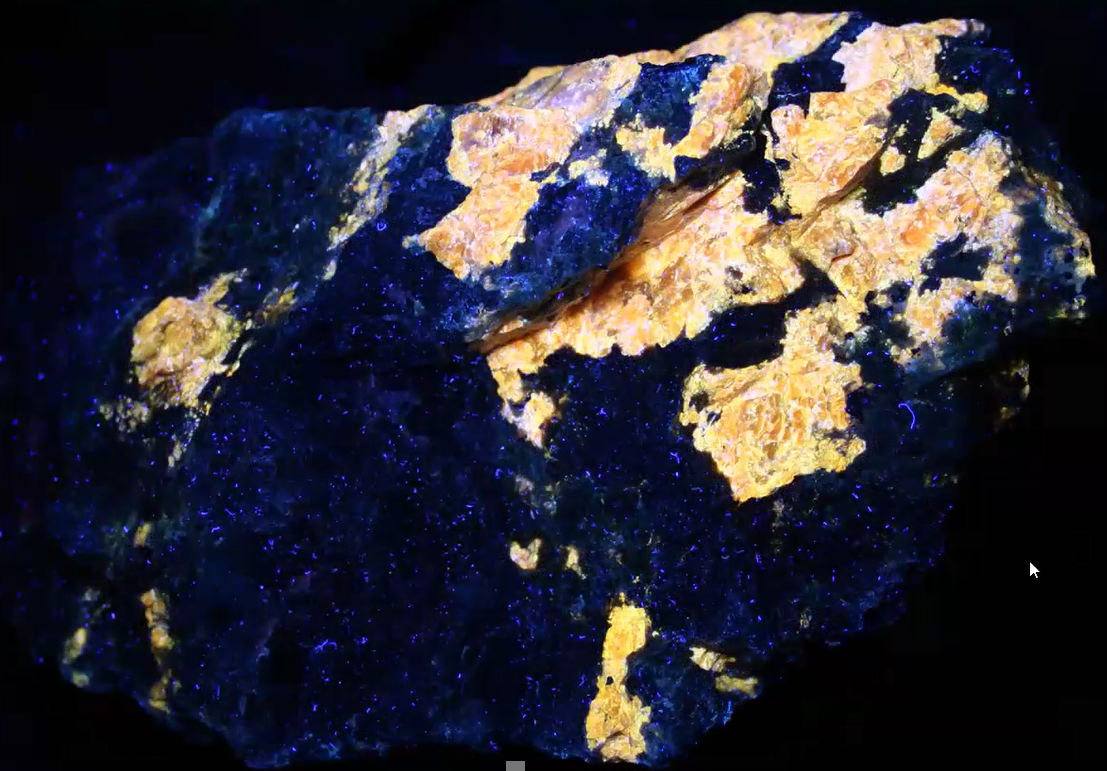
As with many of the specimens Jim showed his MSDC audience, the longwave UV LED flashlights are more effective in bringing out brighter fluorescence than does the shorter UV wavelengths. The striking golden sphalerite specimen below is a case in point.
Conclusion
Jim ended his presentation by calling attention to the diverse mine-related objects, beyond minerals, which some collectors pursue.

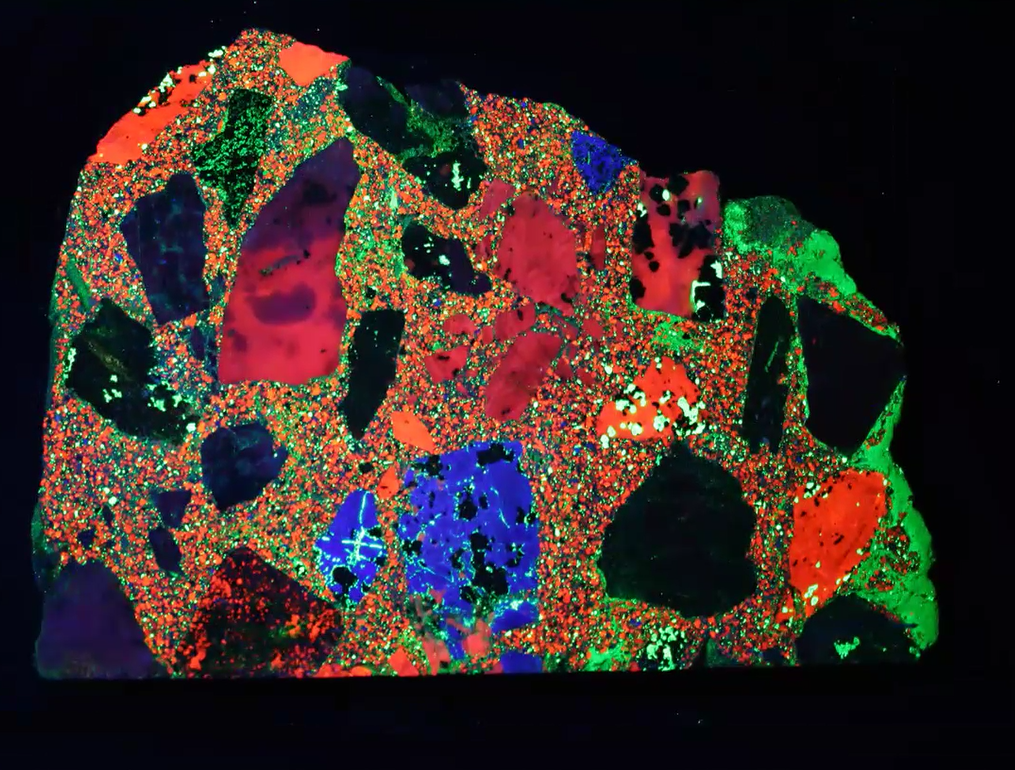
Those mining artifacts range from zinc ingots below to beautiful multicolored concrete composed of rock chip aggregate. And, collectors also look for art work, drill core samples, processed ore, speleothems, and lapidary, all of which fluoresce under UV light as shown in the multicolored concrete below.

Concrete made with fluorescent aggregate and sand:
Question and Answer Session
Kenny Reynolds thanked Jim for his excellent presentation, to which Jim’s audience added their grateful and enthusiastic applause. Kenny then opened the floor for a question-and-answer session.
Perhaps the most basic query which arose earlier in chat posts was: “what causes the fluorescence?”
Jim said that could be the topic of his next 45-minute presentation. But his short answer was that fluorescence is a change in the frequency of the UV light that was initially directed into the minerals and then bounces back out at a different wavelength (and color). Jeff added that the incoming UV light strikes the minerals’ electrons, knocking them into a higher energy electron orbit which results in their emitting energy as a change of color as the electrons drop back toward their original orbit. Jim appreciated Jeff’s explanation.
Members asked Jim if he had a preference between collecting at the two sites and Jim said he preferred collecting at Sterling Hill because of its diversity of mineral specimens that can be found there by cracking large rock with a sledge hammer. Franklin has some hills that are difficult to climb and calcite is the most abundant mineral to be found there.
To learn more from Jim, go to his video where you will learn about finding zincite crystals, the pros and cons of daytime and night collecting, which UV flashlights are preferred, the advantages of UV filtered glass in flashlights, the value of the mine tours, and the importance of getting the events calendar.
Readers: We encourage you to watch the video tape of Jim’s excellent presentation. The slides are stunning. Here is the link: Collecting Franklin Fluorescent Minerals – James Van Fleet (52 minutes).
With no further questions, Kenny again thanked Jim for his superb presentation and wished everyone happy holidays.
