“The Many Faces of Fluoride,” April 2019 Program presented by Herwig Pelckmans
(from Mineral Minutes of May 2019, photos & links added)
by Andy Thompson, MSDC Secretary

Yury Kalish, MSDC Vice President for Programs, introduced Herwig Pelckmans as an international mineral collector from Belgium where he serves as president of the Antwerp mineral club. Herwig said he was visiting the U.S. to make presentations at several mineral conferences. This evening’s presentation to MSDC at the Smithsonian Natural History Museum was the first of three stops along his journey, with speaking engagements at the Atlantic Micromineralogists’ Conference in Alexandria, VA and Rochester Mineralogical Symposium. As a life-long collector and serious student of mineralogy, he has spoken on a wide range of mineral topics, with tonight’s being fluorite and its diverse crystal structures.
Herwig began by noting that a visit to mindat.org, with its 22,500 photos of diverse fluorite crystals found in over 10,000 localities, raises the question of why there are so many different types of fluorite crystal shapes and colors. Specifically, his presentation focused on two questions: what exactly characterizes the structure of these different crystal forms of fluorite and how do those diverse shapes come about?
By way of a brief historical review of this mineral, Herwig noted that Agricola, the famous father of modern metallurgy, in the early 1500s, used the Latin word “fluores” to identify what today we call fluorite. By 1797, chemist Carlo Napione had named it fluorite, supporting Agricola’s recognition that the mineral is well-known for its use as a flux in enabling iron ore to melt at lower temperatures and increase iron’s fluidity. The speaker suggested not only that the name “fluorite” derived from the fact that the mineral increases fluidity in processing iron ore but he also implied that because fluorite, also known as fluorspar, responds to U.V. light, the word “fluoresce” was subsequently applied to any mineral that similarly responded to U.V. light.

On the Mohs scale, its hardness level is 4, confirming what early jewelry makers knew – that it was too soft to serve as a gemstone. So beyond its industrial usefulness, it fell to serious mineral collectors to explore the crystal structure of fluorite.
Due to scientific advances since the above early chemists, we now know that fluorite is a composite mineral made of one calcium and two fluorine ions, CaF2. Because the element fluorine, F, does not exist as a solitary element by itself in nature, its existence as an element was slow to be recognized. Today it is clear that fluorine has always had an abundant presence as an ion in combination with other elements such as iron, copper, silver and gold, including with calcium as in calcium fluorite (CaF2), the subject of tonight’s presentation.
Antoine de Lavoisier (1789) was the first scientist to speculate about the existence of fluorine as a single element. But it would take another century until Henri Moisson (1886) synthesized fluorine gas and the element fluorite was given its rightful place on the Periodic Table with 9 as its atomic number. For that discovery he was awarded the Nobel prize in chemistry in 1906.
Herwig asked his audience: “What color is fluorite?” Collectors know it is found having a wide diversity of colors. But he surprised many by answering: “It is colorless,” referring to fluorite in its pure state. The color, he said, comes from impurities and inclusions in the crystal structure. Similarly, the phenomenon of fluorescing was so named because fluorite minerals exhibit that quality, except for pure fluorite which does not react to ultraviolet light. For collectors particularly interested in fluorescent specimens, Herwig recommended a Belgium colleague’s website, www.fluomin.org as having a wealth of information on fluorescent minerals.
With that reference to crystal structures, Herwig segued into the heart of his presentation, the isometric cubic system of fluorite and its seven basic forms. Starting with its most simple and most prevalent form as a cube, he pointed out that this hexahedron shape has six faces, eight corners, and 12 edges.
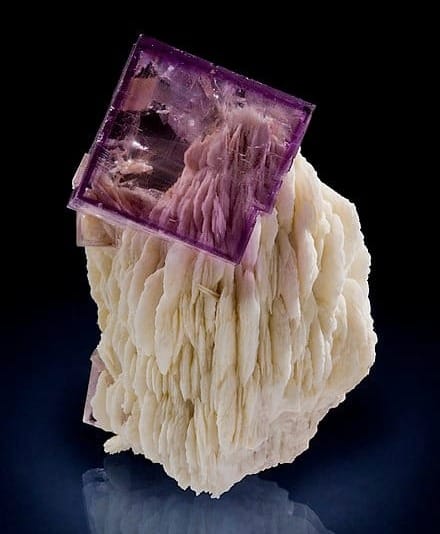
Crystal growth takes place the fastest at their corners and slowest on their faces. Depending on the rate of the crystals’ growth, it can develop multiples of the faces, corners and edges. Those modifications result in fluorite having six additional basic structures beyond that of the simple cube. Herwig used photos of specimens exemplifying each of those distinct structures and drawings to highlight the faces, corners and edges. Those additional six structures included shapes as an octahedron, the rarely found dodecahedron, cuboctahedron, tetrahexahedron, trapezohedron, trisoctahedron and hexactahedron.

In summary, those seven crystal shapes have respectively the following number of faces: 6, 8, 12, 24, 24, 24, and 48. When a collector examines a multi-faced fluorite specimen, it may not be obvious which of the six basic complex crystal shapes is present. So serious collectors often sketch their specimen to map its faces, corners and edges, thereby exposing its true structure.
He then showed unusual variations of fluorite specimens including phantoms in which a crystal formation stopped at some point in its growth and was then overtaken by new growth which encapsulated the early crystal. One striking example was the “Green Spiderman Mystery” in which an original green cube crystal stopped its formation which was then engulfed by a translucent second crystal growth.
Another odd variation was that of a specimen having one edge which grew into multiple edges. When that happens, the number of crystal-faces and edges can be much higher than is found in the above seven basic crystal structures. As the number of sides increases toward 98, the specimen takes on a rounded shape. He showed a photo of a specimen holding the world record of 338 faces and its accompanying drawing which was made in 1908.
He also showed examples of fluorite images having the appearance of common objects such as an egg sunny side up, a cocoon, eyeballs and a perfectly rounded pearl perhaps made by ejection from a volcano. More exotic forms included a hollow stalactite cylinder, an Aztec-like stacked angular structure which, he said was one crystal. Specimen shapes also included a botryoidal bubble, spaghetti stalks, a Christmas tree, Buddha, and a cork screw, to name just a few. Herwig concluded by recommending two books, Fluorite and Crystal Forms of Fluorite.

Dave Hennessey thanked Herwig for his excellent presentation which received abundant grateful applause. Dave also thanked those MSDC members who brought snacks for the post-presentation socializing and thanked members who brought in their own fluorite specimens for an informal, post-presentation show-and-tell sharing.
