Origin of the Phrase: "In the Limelight"
by Ken Rock, MSDC Newsletter Editor

We’ve all heard of being “in the limelight,” which refers to being the center of attention. But not many know that the term has nothing to do with the green citrus fruit we use in our margaritas. The “lime” in limelight refers to a chemical compound, calcium oxide, also known as quicklime. It appears as a white crystalline solid at room temperature, is easily obtained through the thermal decomposition of limestone (e.g., in a kiln).

Limelight was a type of stage lighting once used in theaters and music halls. It refers to an intense illumination created when hydrogen and oxygen are burned to create an extremely hot flame that produces an intense white light, dubbed limelight, when applied to quicklime. The light is produced by a combination of incandescence and candoluminescence.
Limelight was used as stage lighting for years after being discovered by Goldsworth Gurney in the 1820s. Gurney was employed by the Surrey Institute as a lecturer in chemistry and philosophy and did a bit of experimenting of his own on the side. He developed an “oxy-hydrogen blowpipe” which works by introducing a jet of oxygen and hydrogen to a flame. Gurney found that introducing a small chunk of lime to the flame resulted in a blinding white light that could be visible for miles.
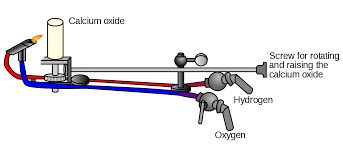
It was after this that Thomas Drummond, a Scottish military engineer, learned about Gurney’s work and thought that the bright light would be useful in surveying. Drummond, who is often credited with inventing the light (so much so that it’s sometimes called “Drummond Light”), used limelight as a means to see in the often dark and stormy mountainous peaks atop Ireland’s mountains, the location of his surveying project at the time. With a limelight at the top of a mountain, Drummond reported that he could see the illumination from as far as 68 miles away. When landmarks and reference points were lit with Drummond’s bright light they could be observed from great distances by surveyors, allowing more accurate measurements to be taken.
In the days before electricity, a bright white light was something many people wanted to get their hands on. It was particularly alluring to the theatre. Limelight was first used in a public theatre in 1837, where the technology was employed at Covent Garden in London. By the 1860s, the use of limelights in theatres was widespread. Before this, theaters were typically lit by gas lights which were particularly dim. To make up for it, hundreds of gaslights were used to light a theatre. As you can imagine, this resulted in a huge fire risk.

By the second half of the 19th century, theaters regularly used this powerful form of light which could be focused into a beam to spotlight specific actors or an area of the stage. The light also could be used to create special effects such as sunlight or moonlight. Clearly, actors who were the center of attention on stage being said to be in the limelight.
A downside to limelight was that each light needed to be monitored and adjusted to the block of lime as it burned. Not surprisingly, this type of lighting also proved to be a fire hazard. In 1879, Thomas Edison demonstrated the first practical electric light bulb and by the end of the 19th century most theaters had switched from limelight to electricity, which was safer and more economical. Nevertheless, this development didn’t signal lights out for “in the limelight,” which remains a common expression.
Although it’s no longer used to help provide illumination, quicklime does serve some practical industrial uses because of its high melting point. Specifically, it is used in iron and steelmaking, where it is added to the furnaces to remove impurities from the produced metal. It is especially effective in removing phosphorus, sulfur, and silica, and, to a lesser extent, manganese. Calcium oxide (and calcium hydroxide) is also an important chemical for raising the pH of potable water and wastewater during its treatment. This property also makes quicklime useful in the pulp and paper industries.

Quicklime is also an important chemical for interacting with soil. One aspect of this is agricultural lime which is added to crops to provide nutrients and control the pH so plants can more easily absorb nutrients in the soil. Another aspect is soil stabilization, a process by which quicklime and hydrated lime are added to soils to render them suitable for load-bearing applications, such as highway construction. Read more at the ANSI Blog: Calcium Oxide: From Ancient Warfare to Modern Industry https://blog.ansi.org/?p=7122
